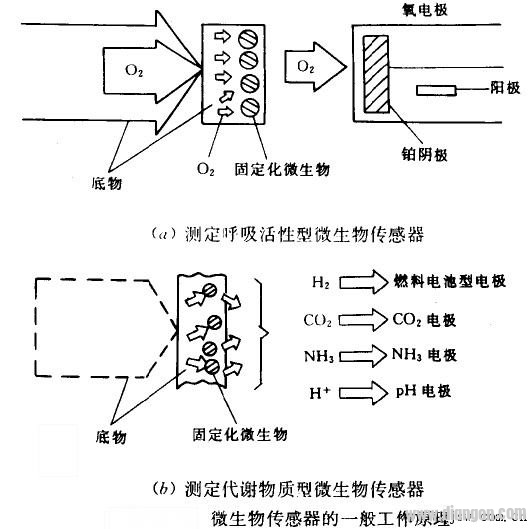
Microbial sensors are based on the interaction between microorganisms and a target substance, and they can be categorized into two main types. The first type involves aerobic microorganisms as sensitive materials. When these microbes come into contact with an organic compound, their respiratory activity increases. This change in respiration is measured to detect the presence and concentration of the test substance, forming what is known as a respiratory-active microbial sensor. The working principle is illustrated in (a). In this setup, aerobic bacteria are immobilized on a diaphragm-type oxygen electrode, creating a microbial electrode. When this electrode is placed in a solution containing an assimilable organic compound, the compound diffuses into the gel matrix containing the microbial cells. As the microorganism metabolizes the organic matter, its respiratory activity increases, leading to a reduction in the amount of oxygen that reaches the oxygen probe. This decrease in oxygen levels results in a drop in the oxygen current, which is used to indirectly determine the concentration of the organic substance. Because of this mechanism, this type of sensor is typically classified as a current-based microbial sensor. The second type of microbial sensor uses an oxy-oxygen-sensitive material. Instead of measuring respiratory activity, it detects specific metabolites produced by the microorganism during the assimilation of organic compounds. This approach leads to the development of a metabolite-based microbial sensor, as shown in Figure 12-36(b). If one of the small molecules produced during metabolism is the target substance for the electrode, the electrode acts as a signal transducer. Combined with a microbial membrane, this forms a complete microbial sensor that can measure the concentration of the target compound. For example, hydrogen-producing bacteria can be embedded in a polymer gel and placed on a fuel cell. In this system, the bacteria generate hydrogen gas, which is then oxidized at the anode. The resulting current is proportional to the amount of hydrogen produced, which in turn reflects the concentration of the organic compound in the sample. This method allows for real-time detection and quantification of substances in solution. In another configuration, the microbial electrode is immersed in a phosphate buffer solution (pH 7.0), where the anode contains an active material such as hydrogen. During the oxidation process, an electric current is generated. The organic compound from the sample diffuses into the hydroquinone layer within the gel film, where it is metabolized to produce hydrogen. This hydrogen then diffuses to the anode, where it is oxidized, producing a measurable current. The magnitude of this current is directly related to the concentration of the organic compound in the sample, allowing for accurate and efficient analysis. Infrared Optical,Zinc Selenide Lens,Optical Zinc Selenide Lens,Optical Imaging Lens Danyang Horse Optical Co., Ltd , https://www.dyhorseoptical.com